Contract Development Services
Our full-time, U.S.- based team of engineers consists of medical device design engineers, project managers, and manufacturing engineers skilled in the art and science of medical device design and development. Whether your organization is in stealth mode, a startup, or a well-established multi-national, we have an expansive service offering you can leverage. Through careful review of the most recent FDA guidelines and regulations and ISO standards our staff applies its vast knowledge to help accelerate project timelines and reduce risk. We pride ourselves on customer focus and transparency.
Product Design Services
We offer product design services from prototype through transfer to mass production. We base our design inputs on your needs, risk assessment, and regulatory requirements. We verify and validate designs through bench, lab, and user testing, and we use prototyping and testing to improve design specifications. Once design is complete, we develop and validate processes to help you scale for commercialization.


Design for Manufacturing & Assembly Services
We focus on design for manufacturing efficiency while also considering labor and materials costs, lead time, and safety. We offer a variety of assembly techniques including:
- Mechanical assembly
- Adhesive bonding
- Ultrasonic welding
- RF Welding
- Laser Welding & Soldering
Your custom medical devices can also be laser marked, chemical etched, or hot stamped as part of the final device marking process. We ensure all finished devices go through a rigorous inspection prior to packaging.
Testing Services
We offer a wide range of testing services from tensile testing to leak-and-flow testing. We can develop custom test procedures or work with outside labs to perform standardized testing.
We’re able to provide appropriate studies establishing SAL (sterility assurance level), Bioburden, Non-Pyrogenicity, nontoxicity, package integrity and EtO residuals, as well as bacteriostasis and fungistasis for standard products. We can also collaborate on your custom needle requirements to address testing and validation needs for their specific product requirements.


Validation & Verification Services
Our team develops Verification & Validation master plans and supporting protocols, and we manage execution of those activities using both internal and external resources. Beyond that, we pack and palletize to customer specifications and coordinate shipping to designated third-party sterilization sites.
Finishing & Packaging Services
Our finishing services can help you bring your product to market faster.
- Polishing
- Corrosion resistance
- Device coatings
- Packaging
- Sterilization
For low-volume operations, our team can hand pack your product into Tyvek® pouches that we then can label and sterilize.
Our state-of-the-art MULTIVAC packaging solution is a great option for high-volume components or finished goods. We can configure our MULTIVAC to incorporate Tyvek or paper, depending on your sterilization process objectives. Both options ensure that your product coding and marking is compliant with UDI requirements for traceability.

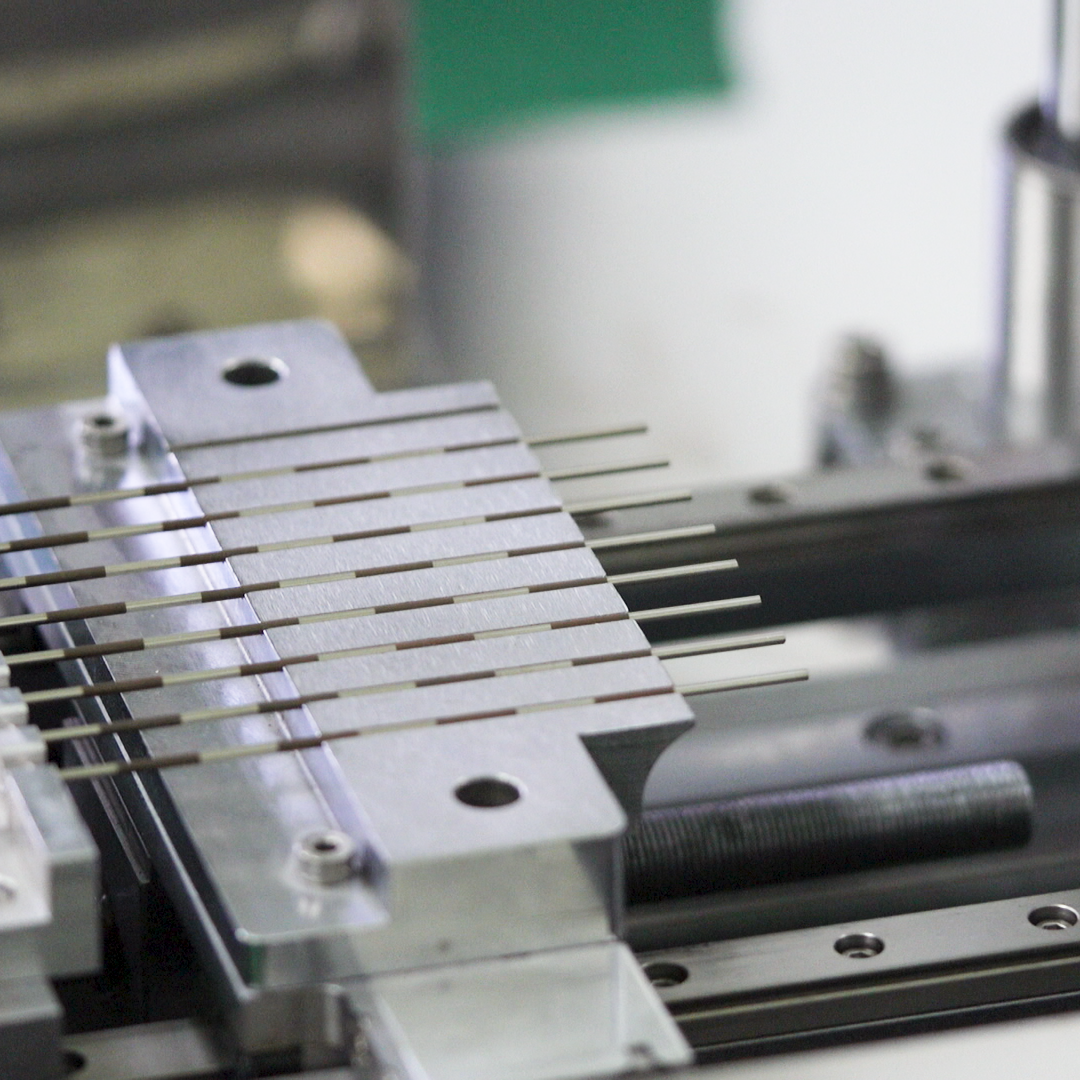
Rapid Prototyping Services
We manage outside sources to turn designs into prototypes with turnarounds times ranging from days to weeks.
Voice of Customer Collection
We can help gather the voice of the customer feedback in support of device design, usability, preference, etc.


Leak Testing of Designs
We offer pressure decay leak testing using custom or off-the-shelf leak testers with custom interfaces to each device.
Ability to Leverage our QMS
We have a robust and thorough QMS. Depending on the situation, we could potentially leverage our QMS in support of your process or provide expert guidance on the requirements and process.


